CRF Research Efforts
The Choroideremia Research Foundation (CRF) has provided over $6 million for over 100 research grants to help find treatment options and a cure for choroideremia (CHM).
If you are interested in submitting a request for funding, please go to the Apply for Funding section of our website for information.
View list of grants awarded as Word document (.docx)
Clinical Trials, Endpoints and Natural History
Clinical Trials
While there is no treatment or cure for choroideremia, there are clinical trials underway testing potential treatments for CHM. Individuals interested in being part of a clinical trial or being treated for CHM when a treatment or cure becomes available will need to have had a genetic test to confirm their diagnosis of choroideremia. Please visit our information for free Genetic Testing.
Please note that any information regarding clinical trials or genetic testing is provided for informational purposes only. The Choroideremia Research Foundation does not endorse any specific company, clinical trial or genetic test. Please discuss any questions you may have with your healthcare provider.
A number of clinical trials and natural history studies for choroideremia are currently available and listed on the National Institutes of Health’s Clinical Trials website.

Click here for basic information on clinical trials.
Click here for information for patients and families.
Endpoints
More recently, however, new and improved outcome measurements have been identified and are now recommended for CHM trials. These endpoints include test results related to scotopic microperimetry (night vision and light adaptation), fundus autofluorescence (retinal health and function), mobility testing and other functional and imaging assessments.
Natural History Data
The Rare Disease Cures Accelerator Data and Analytics Platform (RDCA-DAP) has now added two natural history datasets for choroideremia to their database. This request was informed by extensive discussions between RDCA-DAP and the Choroideremia Research Foundation, identifying the critical need to build a comprehensive natural history of the disease and make that information publicly available through their platform to international scientists. The two studies include:
Efficacy and Safety of BIIB111 for the Treatment of Choroideremia (STAR)
Data Contributor: Biogen
Study ID: NCT03496012
Sponsor ID: NCT03496012
Natural History of the Progression of Choroideremia Study (NIGHT)
Data Contributor: Biogen
Study ID: NCT03359551
Sponsor ID: NCT03359551
To access this data, please create an account here: https://portal.rdca.c-path.org/
More information about this collaborative effort can be found here:
Join the International Choroideremia Research Network (ICRN)
The ICRN is a global collaboration of multidisciplinary researchers who are accelerating knowledge about CHM pathophysiology, developing therapeutic options, and striving to improve patient outcomes.
CHM Eye Donation
Donate Your Eyes for Sight-Saving Research!
As an eye donor, gifting your eyes for choroideremia (CHM) research after your death is a heroic act. By studying this donated tissue, researchers will better understand the disease pathology and strive to develop new treatments and a cure for CHM.
To facilitate this effort, the Choroideremia Research Foundation has initiated a new collaboration with Eversight, a nonprofit organization whose mission is to restore sight and prevent blindness through the healing power of donation, transplantation, and research. Via Eversight, male CHM patients and female CHM carriers can donate their eyes for research in the states of MI, IL, OH, NJ and CT. Eye donation in other states or countries may be arranged through local eye banks that are members of the Eye Bank Association of America and the Lion Clubs International Eye Banks.
Together, we can deliver retinas affected by CHM to the researchers who need them, such as Malia Edwards, PhD, Assistant Professor of Ophthalmology at Johns Hopkins Medicine in Baltimore, Maryland.
Dr. Edwards states, “It is impossible to overemphasize the value of eye donations to research. While animal models and cell culture experiments can help us understand choroideremia, these do not compare to the knowledge we gain by investigating eyes from CHM patients. Eye donations allow scientists to study changes to individual cells and types of cells, helping us understand why vision is lost. Donations from carriers are particularly crucial because the milder vision changes they may experience will help explain disease progression of CHM even more than looking at the late stage of the disease in eyes from CHM patients. Through eye donation, all those affected by CHM can help us understand this devastating disease which will lead to improved treatment and impact the lives of future patients.”
Eyes can be donated for CHM research regardless of age, race, or medical history. Some people wonder if their religion is opposed to eye donation. Rest assured that most faiths support these donations as the ultimate act of charity. It is also important to remember that eyes are typically removed and disposed of by funeral homes for open-casket funerals and of course lost when cremation is chosen. Check with your minister, pastor, rabbi, imam, or other religious leaders if you have questions.
There are several steps you will need to take if you wish to donate your eyes for CHM research:
- Once an individual decides they would like to donate their eyes for CHM research, they should notify their family of their wishes.
- The donor should contact CRF to let them know they are interested in donating their eyes and complete both the CHM Eye Donation Consent Form and the CHM Eye Donor Wallet Cards. Next-of-kin cooperation with a medical/social history interview is required before donation, so it is helpful if your family and friends know how you feel about donation.
- Upon the donor’s passing, family should notify both CRF at email hidden; JavaScript is required and their nearest Eye Bank, which can be found at Eye Bank Association of America and through Lions Clubs International.We understand that losing a loved one is a most stressful and sorrowful time; however, to ensure that these important retina cells remain viable, recovery must take place as soon as possible. It is important to remember that the donation of eyes needs to occur as soon as possible after death, ideally no more than 8-12 hours. The faster the eyes are received, the more information can be gained from them.
Please note that donating your eyes for CHM research cannot be accomplished simply by signing the organ donor information portion of your driver’s license. Eye donation for CHM research is a separate process that can only be accomplished by working with CRF and Eversight or your local eye bank.
To learn more about becoming a CHM eye donor, contact CRF at 800-210-0233 or email hidden; JavaScript is required, and you can get further explanation as to how you can make your eye donation wishes known.
By becoming a choroideremia eye donor, you can be proud knowing you are helping to improve the quality of life for all CHMers now and in the future.
CHM Evolving Therapies
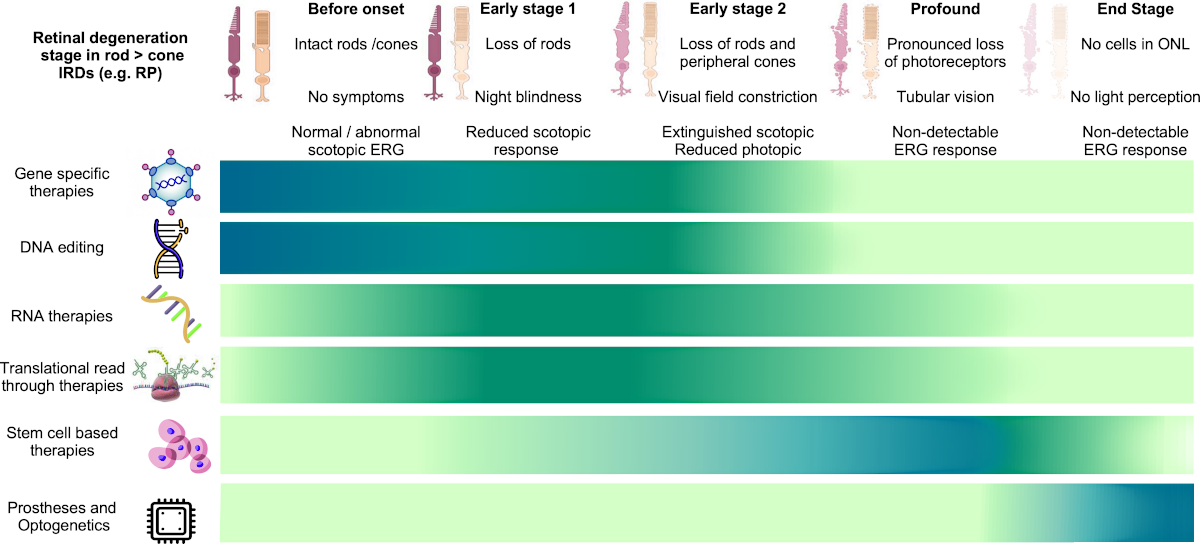
Approach with care. In our quest for a cure, foundational knowledge of CHM variations, their progressions and outcomes will be key. From there, we’ll need multiple research approaches to achieve our goal. Today, those range from gene therapy trials, DNA editing, and transplantation to stem cell therapy and more. While ongoing discoveries keep us confident, it’s the support from our collaborators that keeps us going.
A summary of Evolving Therapies and the stage(s) they target (darker color) is shown in this graphic. More information on each therapy is detailed below this chart.
Citation: Schneider, Nina & Sundaresan, Yogapriya & Gopalakrishnan, Prakadeeswari & Beryozkin, Avigail & Hanany, Mor & Levanon, Erez & Banin, Eyal & Ben-Aroya, Shay & Sharon, Dror. (2021). Inherited retinal diseases: Linking genes, disease-causing variants, and relevant therapeutic modalities. Progress in Retinal and Eye Research. 89. 101029. 10.1016/j.preteyeres.2021.101029.
Dark blue color shading on the table indicates the potential therapy is most suitable for this level of progression
Light green color shading on the table indicates the potential therapy is less suitable for this level of progression
CHM Variants
Sarkar, H., & Moosajee, M. (2022). Choroideremia: molecular mechanisms and therapies. Trends in Molecular Medicine.
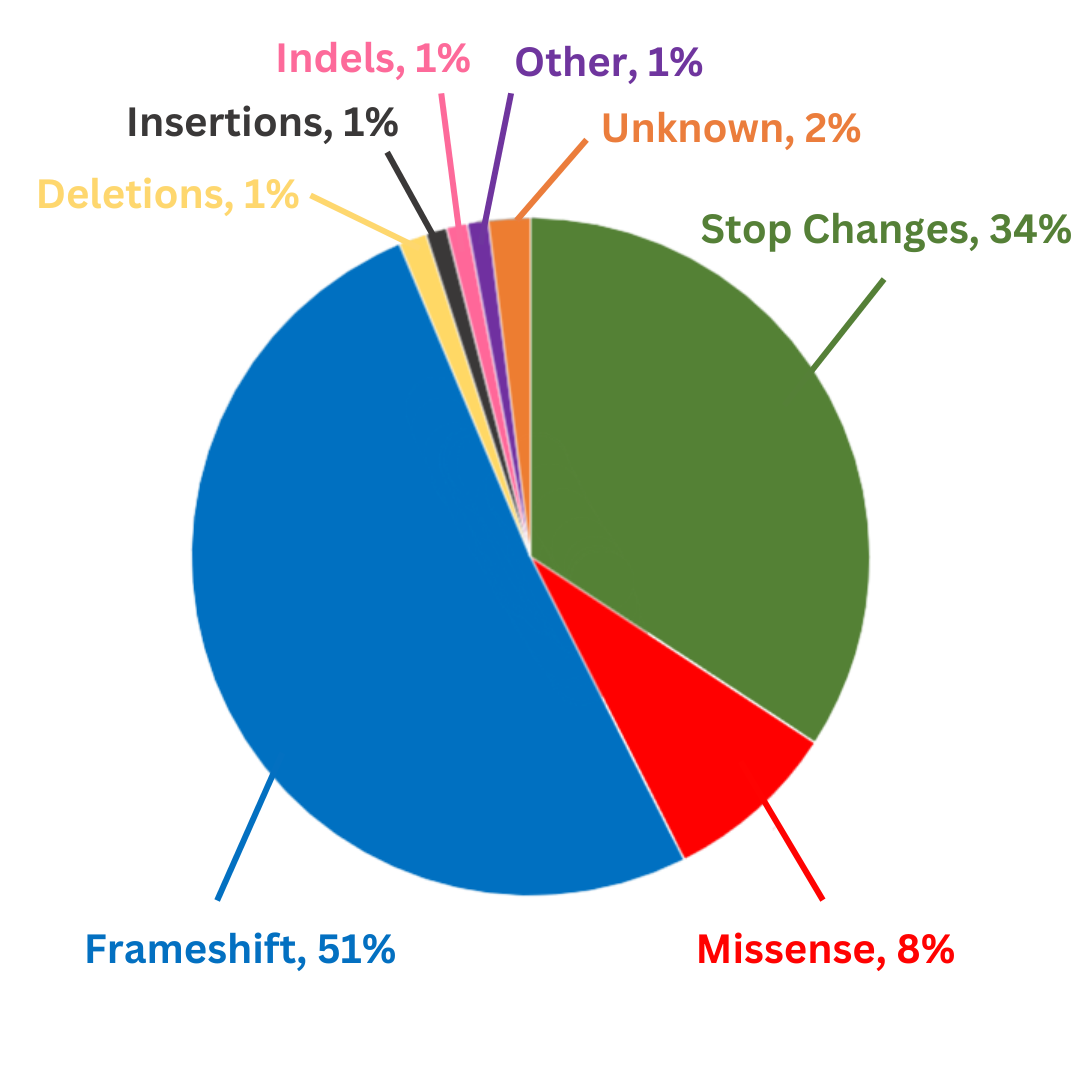
Knowing a patient’s genetic testing results, including what type of genetic variant has caused the CHM mutation, may make patients eligible for future targeted therapies that are currently in development. Genetic variants that cause CHM can include nonsense, missense, frame shift, insertion, deletion, or other changes to the gene.
Three hundred unique pathogenic CHM variants have been reported on LOVD (Leiden Open Variation Database). Of these, 51% are frameshift, 34% are nonsense, 8% are missense; 2% are in-frame deletions, 1% are in-frame insertions, and 1% are in-frame indels. Others include variants where no protein is produced, silent changes, and deep intronic; 2% are unknown.
Gene-Specific Therapies
Genetic diseases, like choroideremia (CHM), are caused by a variation, also known as a mutation, or defect, in the body’s DNA. These genetic mutations prevent the body from producing a beneficial protein called REP-1 necessary for certain cells to survive. Gene therapy is a type of treatment for genetic diseases in which the normal gene is delivered into the affected cells, enabling the cells to produce this vital required protein. Gene therapy aims to restore normal cellular function. The delivery of this therapy is achieved using a vector, which is a cell that has been engineered to transport the desired genetic protein and insert it into the targeted cell that needs help. In CHM, without REP-1, photoreceptors and retinal pigment epithelium (RPE) cells will slowly weaken and die. More details regarding the three types of vectors is available below.
1. Natural Virus Vectors
Scientists first tapped into the ability of naturally occurring viruses, like adeno-associated viruses (AAV’s), to serve as the transport/delivery vehicle of the gene to targeted cells in the human body. Certain AAV’s have been modified to prevent them from causing disease in people while still maintaining their ability to enter into the target cells. These modified AAV vectors have been engineered to carry a normal copy of a given gene and deliver it into those cells to restore their normal function and health. In fact, the first FDA approved gene therapy (Luxterna) for a form of retinitis pigmentosa (RP), utilizes a modified AAV2 vector. The first CHM gene therapy trials (coordinated by Nightstar/Biogen and Spark Therapeutics) have also utilized an AAV2 vector modified with the CHM gene. In two previous clinical trials, the CHM AAV2 vector was delivered through a subretinal injection into the back of the eye. This type of delivery aims to provide the vector directly to the targeted area of affected retinal cells. The vector can only reach cells within the specific surrounding area from the injection point. These clinical trials are continuing to collect long-term data to assess the safety and the effectiveness of gene therapy for treating choroideremia patients.
2. Next Generation AAV Vectors
There are a limited number of naturally-developed AAV vectors. Each one has certain properties, characteristics, limitations and challenges to effectively reach and deliver the needed therapy. To overcome these hurdles, Researchers are working to design new and improved AAV vectors through directed evolution or therapeutic vector evolution. The CRF has partnered with 4D Therapeutics to create a novel CHM gene therapy vector that could have the potential to reach more retinal cells through a delivery by an intravitreal injection in the front of the eye. This trial is in its early stages.
3. Non-viral Vectors
There are also a number of possible non-viral vectors that are being developed to deliver gene therapy or other therapies that might offer a reduced immune response than viral vectors. The non-viral vectors are naked DNA, particle based and chemical based. Researchers are working to improve delivery efficiency, which is the major hurdle for almost all of the non-viral vectors.
Additionally, as an alternative to subretinal injections and intravitreal injections, there is new method of administering gene therapies in development, injection into the suprachoroidal space (SCS), which is the space between the sclera and choroid that traverses the circumference of the posterior segment of the eye. This method may offer a more targeted approach to drug delivery with the potential to achieve chorioretinal concentrations 10 times greater than that seen with traditional intravitreal injections.
DNA Editing
DNA, or deoxyribonucleic acid, is the hereditary material in humans and almost all other organisms. Nearly every cell in a person’s body has the same DNA. Genome editing (also called gene editing) is a group of technologies that give scientists the ability to change an organism’s DNA. These technologies allow genetic material to be added, removed, or altered at particular locations in the genome. Several approaches to genome editing have been developed. A recent one is known as CRISPR-Cas9, which is short for clustered regularly interspaced short palindromic repeats and CRISPR-associated protein 9. The CRISPR-Cas9 system has generated a lot of excitement in the scientific community because it is faster, cheaper, more accurate, and more efficient than other existing genome editing methods.
CRISPR-Cas9 was adapted from a naturally occurring genome editing system in bacteria. The bacteria capture snippets of DNA from invading viruses and use them to create DNA segments known as CRISPR arrays. The CRISPR arrays allow the bacteria to “remember” the viruses (or closely related ones). If the viruses attack again, the bacteria produce RNA segments from the CRISPR arrays to target the viruses’ DNA. The bacteria then use Cas9 or a similar enzyme to cut the DNA apart, which disables the virus.
The CRISPR-Cas9 system works similarly in the lab. Researchers create a small piece of RNA with a short “guide” sequence that attaches (binds) to a specific target sequence of DNA in a genome. The RNA also binds to the Cas9 enzyme. As in bacteria, the modified RNA is used to recognize the DNA sequence, and the Cas9 enzyme cuts the DNA at the targeted location. Although Cas9 is the enzyme that is used most often, other enzymes can also be used. Once the DNA is cut, researchers use the cell’s own DNA repair machinery to add or delete pieces of genetic material, or to make changes to the DNA by replacing an existing segment with a customized DNA sequence.
Genome editing is of great interest in the prevention and treatment of human diseases. Currently, most research on genome editing is done to understand diseases using cells and animal models. Scientists are still working to determine whether this approach is safe and effective for use in people. It is being explored in research on a wide variety of diseases, including single-gene disorders such as cystic fibrosis, hemophilia and sickle cell disease.
RNA Therapies
RNA stands for ribonucleic acid and it is an essential component of all living cells. RNA is used for ‘translation’, the process in which proteins are created in a cell. RNA itself is produced from DNA, in a process called ‘transcription’. An RNA therapy is designed to correct the mistake, or mutation, in the RNA of someone with a genetic disease. By correcting the mistake, the RNA can then be used to create the protein that the cell needs, taking away the underlying cause of the disease. RNA therapies can perform the same functions as other types of medicines but have broader applications. If a genetic disease results in a missing or broken protein, an RNA therapy can often correct this. Research has shown promising results and other approved RNA therapies are helping people around the world. Just like gene therapy and CRISPR approaches, RNA therapies are genetic therapies that aim to take away the underlying cause of a genetic disease. However, the fundamental approach is different.
Gene therapy and CRISPR approaches make changes to DNA. These changes are permanent, with the risk of permanent side-effects. Unlike gene therapy, RNA therapies aim to repair RNA, which is a product of DNA, therefore any changes made to the RNA are reversible. RNA therapies are much smaller than gene therapies making manufacturing and delivery easier. RNA therapies work best if they are delivered directly to the affected organ. In the case of retinal diseases, RNA therapies can be injected into the vitreous of the eye, which is a cavity filled with a jelly-like fluid, via intravitreal injection.
Similarly, transfer RNA, or tRNA, as it’s known to biologists, helps cells assemble proteins from amino acids by translating the nucleotide code of messenger RNA (mRNA). Each tRNA molecule reads a trio of nucleotides called a codon, which represents instructions for adding a particular amino acid to a protein. The tRNA then earns its name by transferring the corresponding amino acid to the cell’s protein-making machinery, known as the ribosome. This process repeats along the length of the mRNA strand until the ribosome runs into a stop codon, a special trio of nucleotides that marks the end of the protein-making instruction manual and the ribosome’s job is done.
Translational Read-Through Therapies
Translational read-through therapies are a pharmaceutical approach to addressing the premature stop codon problem noted in the RNA section above. Premature termination codons (PTCs) in the coding regions of mRNA lead to the incorrect termination of translation and generation of non-functional, truncated proteins. As a simplified analogy, think of it as a train headed to a destination with multiple stops. When a PTC exists, rather than moving through the stops to get to the destination, at one of the stops, the train returns to the original station, leaving all the passengers stranded at the other stops. In a PTC, the “passengers” are proteins that are unable to board the train and are left with no place to go.
Translational readthrough of PTCs induced by pharmaceutical compounds is a promising way of restoring the “train” to complete its journey to create functional protein expression and reduce disease symptoms, without affecting the genome or transcriptome of the patient. It is estimated that as many as 30-40% of choroideremia patients may have this premature stop codon mutation, also known as a nonsense mutation. While in some cases proven effective, the clinical use of readthrough-inducing compounds is still associated with many risks and difficulties and research is continuing in this area.
Stem Cell Based Therapies
Stem cells are referred to as progenitor cells, which means they can develop into almost all other cells in the body. Historically, stem cells were only obtained from embryos which created significant controversy. Recent developments, however, have enabled scientists to take a blood or skin sample from an individual and create stem cells from these tissue samples. These stem cells, referred to as induced Pluripotent Stem Cells (iPSCs) can then be influenced to evolve into other cell types in the body by following specific scientific protocols. For CHM research, scientists can use iPSC cells to create any types of specific types of retinal cells, including photoreceptors and retinal pigment epithelium (RPE) cells, which are the cell types lost in choroideremia. In fact, IPSCs are now being used to create CHM retina organoids, which are miniaturized retina structures. These iPSC-derived CHM organoids are now being used to aid researchers in studying the disease process of CHM on a cellular level.
Scientists are working to make these iPSC-derived photoreceptors and RPE cells into transplant patches which could be surgically implanted to replace areas of vision loss.There are two distinct approaches based on the source of the iPSCs.
1. Allogenic (Donor) iPSCs: An allogenic iPSC uses stem cells derived from healthy donors, which can be produced commercially in large amounts and can be assembled with a scaffold layer of RPE cells on one side and photoreceptors on the other side. The scaffold patch is then transplanted into the eye. The primary advantages of this method is that it is not disease specific, potentially benefiting patients with a variety of vision loss disorders, and it is cost effective. A challenge of this method would be the possible rejection of the cellular transplant.
2. Patient-derived iPSCs: In this method, an iPSC is created from the patient’s own cells. The iPSC would need to be genetically edited to correct the disease mutation before the iPSC is differentiated into the needed patch for transplantation. The primary advantage of this method is a reduced chance of transplant rejection. The challenge is the very labor intensive nature of the process and thus an increased cost.
Prostheses and Optogenetics
With advanced CHM and other inherited retinal diseases (IRDs), the retina cells that respond to light and send signals to the brain (photoreceptors) have died; leaving only the neurons that carried their signal to the brain. Optogenetics and prosthetics are working to create some form of a replacement signal that the neurons can still deliver to the brain which can be understood and processed like natural vision.
Prosthetics for vision include man made devices like microchips than be implanted in the eye. The chips receive light and convert it into electronic signals that are sent via electrodes on the back of the chip to the neurons that remain in the back of the eye. Through this process the brain could learn to interpret these new signals as functional vision. Challenges for these implants include limits to the amount of the signals sent because of heat from the chip in the eye. In limited research, patients with prosthetics have had light-sensitivity restored with the ability to distinguish specific objects.
Optogenetics is the technique of converting retinal neurons to respond to light. Using a gene therapy vector to target the neurons and inserting a light responsive protein, like rhodopsin, the neurons can send signals along the optic nerve to the brain. Researchers control the light and images sent to these neurons with special wearable glasses or goggles. The glasses have video cameras and can convert the streaming video into a digital code of pulsating bits of light that is projected into the treated eye. The neurons then respond by sending the coded signals to the brain.
Scientists know that vision is sent as a code. Some trials have created their own code and believe that the brain can adjust to a new signal over time. One trial is utilizing what is believed to be the actual code used by the brain, making adaptation to these goggles much quicker. Some researchers believe that the technique has the potential to cure blindness caused by CHM and other IRDs.
Other Evolving Research
Until such time that a cure or treatment for CHM is found, there are many other areas of research underway attempting to delay the progression of vision loss by improving the overall health of the eye. These include but are not limited to:
- Reducing mitochondrial dysfunction.
- Reducing oxidative stress.
- Providing electrical stimulation to the eye to prolong visual field and acuity.
- Improving phagocytosis. Phagocytosis is a cellular process for ingesting and eliminating excess particles or debris, such as dead cells. Phagocytosis is an essential process for tissue homeostasis, or creating a healthy equilibrium in the eye.
- Utilizing nutritional supplements, a healthy diet and exercise to optimize eye functioning (see Patient Toolkit)
- Research funded in part by CRF has shown that drugs that improve melanin production represent a potential novel therapeutic avenue for CHM.
Still other research is underway to improve assistive devices such as mobile apps to read currency or provide 1:1 personal assistance; smart wearable technology to help with object identification and mobility orientation; and electronic travel aids such as intelligent canes to improve safety and navigation that will help people with vision loss improve their activities of daily living.
CRF is dedicated to supporting and encouraging research for all these potential therapies to address the entire spectrum of vision loss experienced by CHM patients.
CHM Animal/Cell Models
CHM BioBank
The CRF has created an internationally accessible BioBank to provide the basic cellular resources that are needed for CHM research. CHM BioBank resources are housed at WiCell and Coriell. By creating induced pluripotent stem cells (iPSC’s), or cells from choroideremia patients, scientists can study the disease and learn more about its progression at a microscopic level.
In addition, future treatments can be tested on these iPSC’s rather than on animal models of choroideremia which may not respond in the same way as humans.
For a listing of the Coriell CHM resources including iPSC, Fibroblasts, DNA and LCL please click the link below:
WiCell also has select Choroideremia (hiPSC) available. Click below for cell lines.
CHM Animal Models
Before moving into human clinical trials, the FDA may require data demonstrating a proposed therapy is effective in animal studies.
CRF is pleased to share that Dr. Mariya Moosajee has rederived Dr. Miguel Seabra’s original CHM mouse line and has established a live colony at Moorfields in the UK. Dr. Moosajee would be delighted to collaborate with any researchers interested in accessing these mice by shipping them globally via a Materials Transfer Agreement. She also has CHM zebrafish, iPSC, organoids, fibroblasts and DNA available upon request. If you are interested in accessing any of these resources, please contact her directly at: email hidden; JavaScript is required
Additionally, members of the International Choroideremia Research Network would also be willing to share or collaborate with their CHM research resources as needed:
– Dr. David Gamm at the University of Wisconsin has CHM organoids and iPSC and can be reached at: email hidden; JavaScript is required
– Dr. Viki Kalatzis at INSERM in France has CHM RPE, iPSC, fibroblasts and organoids at: email hidden; JavaScript is required
– Dr. Malia Edwards at Johns Hopkins has CHM human donor eye tissue and can be reached at email hidden; JavaScript is required
If you would like to add your institution’s CHM research resources to this list, please email email hidden; JavaScript is required directly with more information.
Several resources exist to provide CHM animal models:
- International Mouse Strain Resource
- Mouse Genome Informatics
- Infrafrontier – mouse disease models
- Mutant Mouse Resource and Research Center
- Zebrafish Information Network
- Porcine IRD Models-Renovate Biosciences
- Premade Adenovirus with ORF of Choroideremia
- Cyagen – Mouse Models
Variant/Mutation Data
Although only one mutation, the CHM gene, is known to cause choroideremia, many different genetic errors can occur to cause this mutation. Here are two resources which are helping to identify and document the variety of mutations which can occur:
Other Research Resources
Google Scholar provides a simple way to broadly search for scholarly literature.
Stone Rounds provides a disease atlas, clinical simulator, resources for learning about IRD’s, group teaching, media, “Success Camp”.
PubMed comprises citations for biomedical literature, life science journals, and online books.
CRF’s CureCHM YouTube Channel has over 225 videos including scientific webinar presentations, interviews with leading researchers, and more.
Science News
CRF is now translating scientific papers into lay summaries so that this information can reach a wider audience.
The links to these PDF summaries are below.

- New findings could improve the development and efficacy of therapies for CHM
- Disease severity in female carriers of choroidermia and a possible association with CHM expression levels
- Rapid Quantification of the Binocular Visual Field for Clinical Trials
- A novel approach to unravelling the pathophysiology of CHM using iPSC-derived RPE from patients
Apply for Funding
Our objective is finding a cure or effective treatment for choroideremia.
Accomplishing this goal relies on:
- Research that provides essential resources and knowledge for the field (e.g. model systems, understanding genetic underpinnings, annotated patient specimens)
- Innovative research that opens new pathways for diagnosis and drug discovery
- Promising projects that are less likely to get traditional funding such as:
- Seed funding for hypothesis-generating projects
- High quality projects proposed by young investigators
- Foundational projects with an important but long-term payoff
Please note there are two different application categories.
The first is for the Randy Wheelock Research Award. This award recognizes emerging scientists and early-career research professionals working on choroideremia (CHM) or related vision loss issues. Eligible recipients will be either doctoral or post-doctoral candidates and professionals, and awards will be granted in the amount of $50,000. Introduced in 2020, the Randy Wheelock Award celebrates the decades-long advocacy of its namesake to find treatment or a cure for CHM. Proposals for the Wheelock Award are due annually on June 30.
2023 Luisa de Lemos, PhD
2024 Shalhevet Izraeli
2025 Alice Y. Zhang, MD
If you are interested in submitting a request for funding please format your application as specified in the Grant Application Guidelines. Applications should be submitted to Kathi Wagner, Executive Director, Jess Thompson, MD, Science Advisory Board and Research Committee Chair and Mike McConnell, PhD, Chief Scientific Officer.